For decades, the foundations of cancer treatment have been surgery, chemotherapy, and radiation therapy. However, these treatments are not always successful—hence, further research paved the way for immunotherapy, specifically, Chimeric Antigen Receptor (CAR) T-cell therapy.
How CAR T-cell therapies work
CAR T-cell therapy has been a huge area of research for over a decade, but it was not until the success of drugs such as Kymriah™ (Novartis) that it emerged as one of the most promising treatments in cancer research today. Basically, CAR T-cell therapy is a way to get immune cells called T cells (a type of white blood cell) to fight cancer by changing the genes inside them in the lab. These ‘engineered’ T-cells are then introduced into the patient so they can find and destroy cancer cells [1].
What should be considered when developing a CAR T-cell program
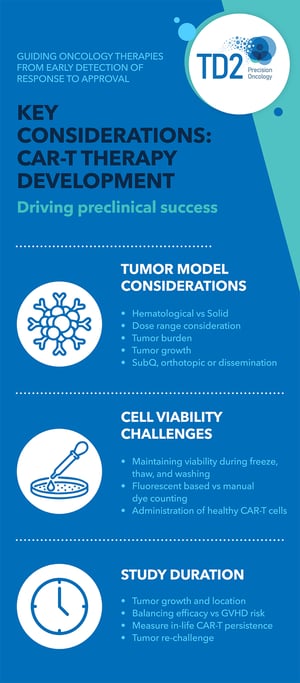
Currently, available CAR T-cell therapies are customized for each patient [2]. Over the past five years, TD2 has been in collaboration with several companies to plan and execute proof-of-concept preclinical studies for the development of a CAR T-cell program. From years of research, our findings provided us with key aspects that need to be considered when developing this program:
- Type of Cancer
The types of cancer that are currently treated using CAR T-cell therapy are diffuse large B-cell lymphoma (DLBCL), follicular lymphoma, mantle cell lymphoma, multiple myeloma, and B-cell acute lymphoblastic leukemia (ALL) [3], with many patients showing complete response to treatment. Although success is exhibited in hematological tumors, it can also be used to treat solid tumors. The key is to understand the growth of the tumor, its location, and the total amount of tumor in the body. Furthermore, dosage also differs between hematological and solid tumors. - State of the Cell
CAR T-cell preparation involves several steps such as freezing, thawing, and washing which could decrease the cell viability. Cryopreserved or fresh cells can be used, and viable cells are sorted and counted before administration. - Study Duration
The length of the study varies depending on the tumor type and location. The possibility of graft versus host disease (GVHD), a condition that occurs when donor bone marrow or stem cells attack the recipient, could also occur at some point. Hence, the study duration must be balanced to consider the possible occurrence of GVHD. The ability of CAR T-cells to eradicate new tumor growth can also be studied.
CAR T-cell therapies are not your average drug development program. With over 75 studies completed, a strong experience in CAR T-cell handling and processing, and the latest technology available for use in in vivo and ex vivo studies, TD2 has increased efficiency and extensive knowledge on executing a reliable CAR T-cell drug development program. Not only do we have the preclinical expertise, but we also drive your program forward through consultative regulatory support and clinical trial management to ensure the best and quality results moving your CAR-T cell therapy to market.
References:
- https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- https://ashpublications.org/bloodadvances/article/4/19/4669/463994/Outcomes-in-patients-with-DLBCL-treated-with
- https://my.clevelandclinic.org/health/diseases/10255-graft-vs-host-disease-an-overview-in-bone-marrow-transplant