Phase I trials test new cancer treatments on a small group of advanced cancer patients to see if the treatment is safe and what dose is best. The goal is to get information on how the treatment is absorbed, metabolized, and hopefully prove it is safe. This information is used to plan larger trials on more patients.
TD2 works with sponsors to plan Phase I trials for their treatments. We have experts who help develop trials from a wide variety of established clinical designs. By selecting the best approach for a sponsor’s new cancer treatment, a trial will provide data on the most appropriate dose range faster, as well as some information on whether the treatment might work, which can help the treatment succeed in the long term.
What is a Model-Based Approach?
Model-based approaches in oncology use mathematical models to simulate how a new treatment might behave in cancer patients, allowing for treatment optimization, drug development, and better clinical trial design. These approaches offer a powerful tool for understanding and treating cancer.
Continual Reassessment Method (CRM) and Dose Escalation With Overdose Control (EWOC)
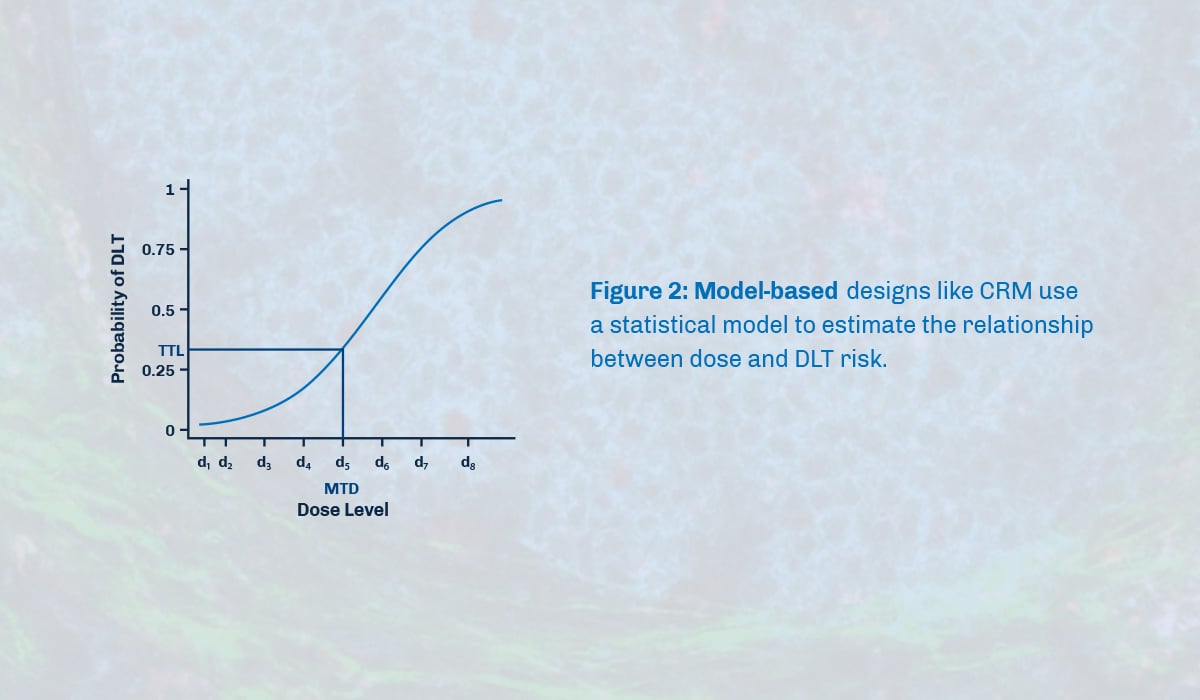
Model-based approaches such as Continual Reassessment Method (CRM) and Dose Escalation with Overdose Control (EWOC) enhance the accuracy of determining MTD (maximum tolerated dose). These designs are based on a statistical model that relies on DLT (dose limiting toxicity) event probability and do not follow a predetermined algorithm, like a traditional 3+3 design. As a result, the dose level for the next patient is unknown until data from previous patients is integrated into the model and design parameters are updated.
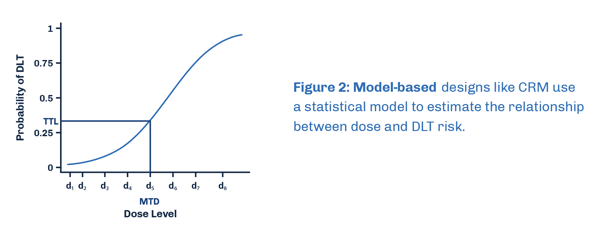
Benefits and Limitations of a Model-Based Approach
Model-based designs clearly define and flexibly choose the treatment goal, resulting in more patients receiving the best dose, less receiving ineffective doses, and providing more information on the highest safe dose and efficacy. Additionally, it allows for easier adjustments to the plan, such as treating different numbers of patients with different doses. However, model-based designs require special software and statisticians to be involved, making them complex for doctors to understand. EWOC is very safe but may not accurately determine the highest safe dose as it tends to be overly cautious.
Considering the Best Approach
The traditional way of developing new drugs involves testing them in stages to see if they are safe and effective. However, in cancer treatment, it has become common to include extra stages to try out different doses of the drug. This can help speed up the approval process if the drug shows promise. There are different ways to design these extra stages, but some are better than others. TD2 works with sponsors to test different doses to find the best one and using other techniques to speed up the process. Our team can help design high-quality studies that use data from earlier tests to find the right dose faster and get new treatments to patients sooner.

WHITE PAPER