Phase I trials are an important step in the development of new cancer treatments. They provide critical information about the safety and optimal dosing of the treatment, which is used to plan larger trials and ultimately determine if the treatment is safe and effective for cancer patients.
One of the approaches TD2 uses in cancer research is a model-assisted approach where experts use statistical models to predict the optimal dose based on existing, known information. By using these models to guide dosing decisions, doctors can improve the safety and effectiveness of the treatment during Phase I trials, thereby setting later phase studies up for success.
What is a Model-Assisted Approach?
The model-assisted approach in cancer treatment trials involves using statistical models to help doctors and scientific experts determine the best dose of a new cancer treatment to give to patients. These trials use rules that were generated by statistical analysis that set ranges of probabilities to predict the optimal dose based on factors such as the patient's age and weight, the cancer type and stage, the class of drug, and information uncovered during preclinical research. By using these models to guide dosing decisions, experts can improve the safety and effectiveness of the treatment during trials.
Modified Toxicity Probability Interval (mTPI)
Modified Toxicity Probability Interval (mTPI) is an example of a model-assisted trial design which helps determine the optimal dose of cancer treatments for individual patients. It analyzes the toxicity data from past patients to calculate the probability of toxicity for a given dose. As new patients are enrolled in the trial, his or her dose is determined based on information already gathered in the trial. This personalized approach helps to improve treatment outcomes and minimize harmful side effects.
Bayesian Optimal Interval (BOIN)
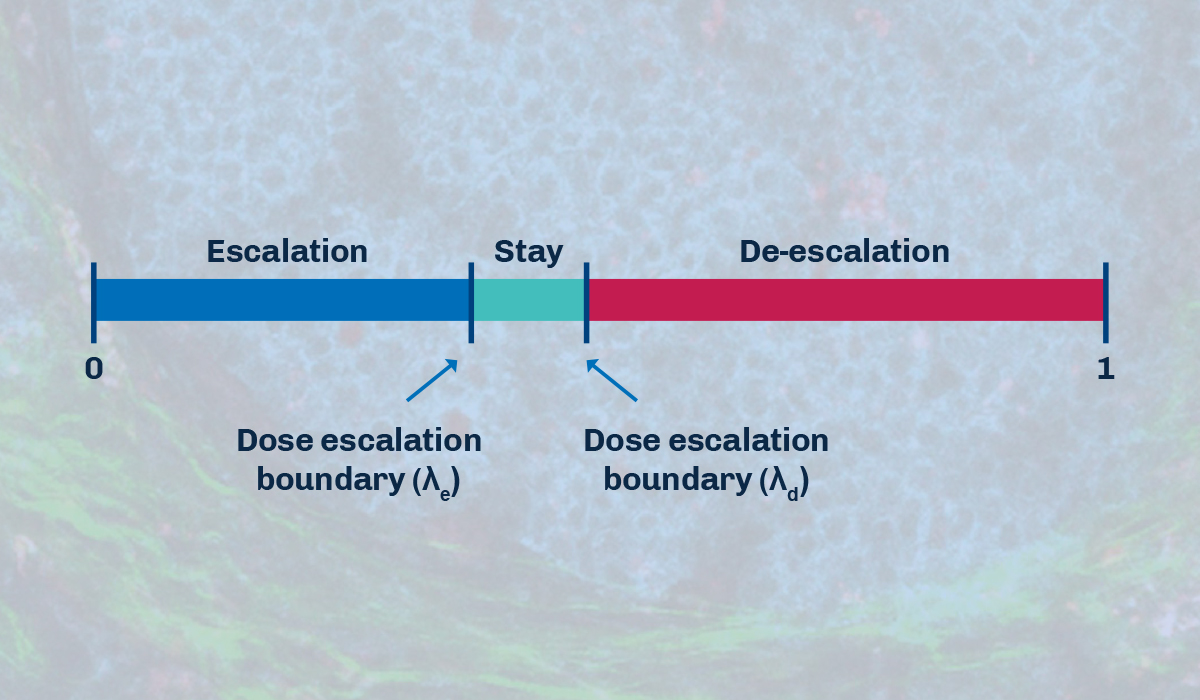
BOIN is another example of a model-assisted trial design. This approach also uses statistical modeling to set several ranges of dose levels for the drug at the onset of the trial. It combines prior knowledge and data obtained from the study to estimate the probability of a patient receiving the optimal dose. BOIN compares the dose limiting toxicity (DLT) rate at the current dose to pre-specified dose escalation and de-escalation boundaries, which are dependent on the target toxicity level (TTL). This method helps determine whether to escalate, stay at the current dose, or de-escalate the dose.

Comparison Between Rule-Based, Model-Based, and Model-Assisted Approaches to Trial Design
Both model-based and model-assisted designs outperform the traditional rule-based trial designs, such as the 3+3 method, in selecting the correct maximum tolerated dose (MTD). A model-based approach requires ongoing statistical analysis from statisticians during the trial as new data is collected. However, model-assisted methods combine the ease of a rule-based trial design by pre-setting dose level ranges at the onset of the trial and the accuracy of the model-based design by using the statistical analysis methods used in model-based designs.

WHITE PAPER