Immunotherapy and cell therapy have revolutionized the field of cancer treatment by harnessing the power of the immune system to fight cancer cells. However, the development of effective immunotherapy drugs such as immune checkpoint inhibitors requires extensive preclinical testing to ensure safety and efficacy. Breakthroughs in the field are faced with several challenges including the generation of appropriate humanized mouse models for preclinical evaluation. Among others, genetically-engineered mouse models have emerged as valuable tools for improving cancer treatment outcomes.
Immune Checkpoints: How Cancer Cells Exploit Them
Immune checkpoints (ICP) are molecules found on the surface of immune cells, including T cells and antigen-presenting cells, as well as on cancer cells themselves. These checkpoints regulate immune responses by either activating or deactivating specific immune pathways which is why they are considered as ‘accelerators’ and ‘brakes’ of the immune system. They maintain immune homeostasis, preventing excessive immune activation that could lead to autoimmune diseases. By carefully balancing immune responses, immune checkpoints ensure that immune cells effectively target foreign pathogens while avoiding self-destruction.
Because cancer cells themselves express ICP on their surface, they can evade the immune system's surveillance. This evasion allows cancer cells to grow, proliferate, and spread without being effectively targeted by the immune system's defense mechanisms.
Fortunately, researchers have made remarkable progress in understanding immune checkpoints on cancer cells, opening the door to innovative treatments. Immune checkpoint inhibitors (ICIs) such as anti-PD-1 and anti-CTLA-4 have been developed to disrupt the interactions between immune checkpoint molecules and their corresponding receptors. These therapeutic antibodies act as "brake releasers," unleashing the full potential of the immune system to mount a robust and targeted attack against cancer cells.
Humanized Mouse Models: Are they good enough?
Humanized mouse models are normally generated through a series of steps to replace the murine immune system with a functional human immune system in a process called xenografting and hematopoietic stem cell transplantation. Although these processes enable the generation of humanized mouse models with immune characteristics, limitations such as overcoming the host’s innate immune response, instability of the human-derived cells, and low levels of the reconstituted cells make them difficult to be used in ICI preclinical drug development.
Genetically Engineered Humanized Mouse Models for ICI Drug Trials
Genetically engineered humanized mouse models have emerged as valuable tools for studying immunotherapy drugs, particularly immune checkpoint inhibitors (ICI). These models address the limitations of traditional humanized mouse models generated through xenografting and hematopoietic stem cell transplantation. Genetic engineering techniques, such as the Crispr/Cas9 technology, have allowed researchers to overcome challenges related to the host's innate immune response, instability of human-derived cells, and low levels of reconstituted cells. By engineering mouse models with single, double, and triple gene knockins for known human immune checkpoint targets, researchers can better simulate the clinical side effects of ICI drugs and evaluate combination therapies or compare different drug candidates. These genetically engineered humanized mouse models provide a more relevant and reliable platform for preclinical evaluation of immunotherapy drugs, improving our understanding of their safety and efficacy.
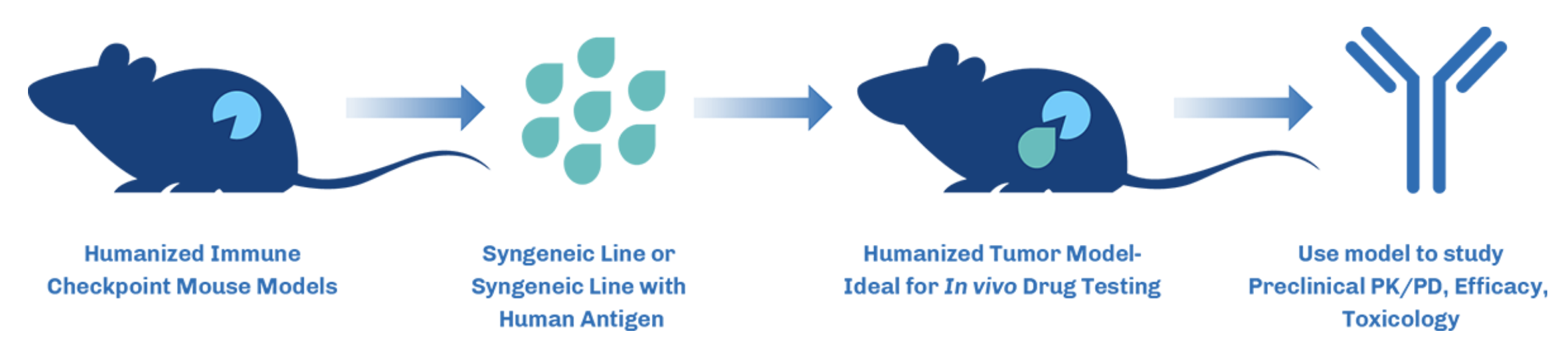
Figure 1. Overview of how humanized mouse models work in Immuno-oncology studies.
Conclusion
Traditional humanized mouse models have limitations in accurately reflecting the complexity of the human immune system and evaluating the efficacy of ICIs. However, through genetic engineering techniques such as Crispr/Cas9, researchers have been able to overcome these limitations by creating genetically engineered humanized mouse models. These models allow for better simulation of the clinical effects of ICIs and enable the evaluation of combination therapies and comparison of different drug candidates. The use of genetically engineered humanized mouse models represents a significant advancement in the field of cancer research and drug development, offering researchers a powerful tool to optimize and accelerate the development of immunotherapy treatments such as ICI for cancer patients.
References
- https://td2inc.com/humanized-immune-checkpoint-inhibitor-mouse-models/
- https://en.gempharmatech.com/product/models_list100139_170104.html
- https://www.genoway.com/commentaries/icp.htm
- Cogels MM, Rouas R, Ghanem GE, Martinive P, Awada A, Van Gestel D, Krayem M. Humanized Mice as a Valuable Pre-Clinical Model for Cancer Immunotherapy Research. Front Oncol. 2021 Nov 18;11:784947. doi: 10.3389/fonc.2021.784947. PMID: 34869042; PMCID: PMC8636317.
- https://www.frontiersin.org/articles/10.3389/frhem.2022.1062705/full#:~:text=However%2C%20this%20model%20has%20some,in%20the%20mice%20after%20the
- Olson B, Li Y, Lin Y, Liu ET, Patnaik A. Mouse Models for Cancer Immunotherapy Research. Cancer Discov. 2018 Nov;8(11):1358-1365. doi: 10.1158/2159-8290.CD-18-0044. Epub 2018 Oct 11. PMID: 30309862; PMCID: PMC8725605.
- Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012 Nov;12(11):786-98. doi: 10.1038/nri3311. Epub 2012 Oct 12. PMID: 23059428; PMCID: PMC3749872.