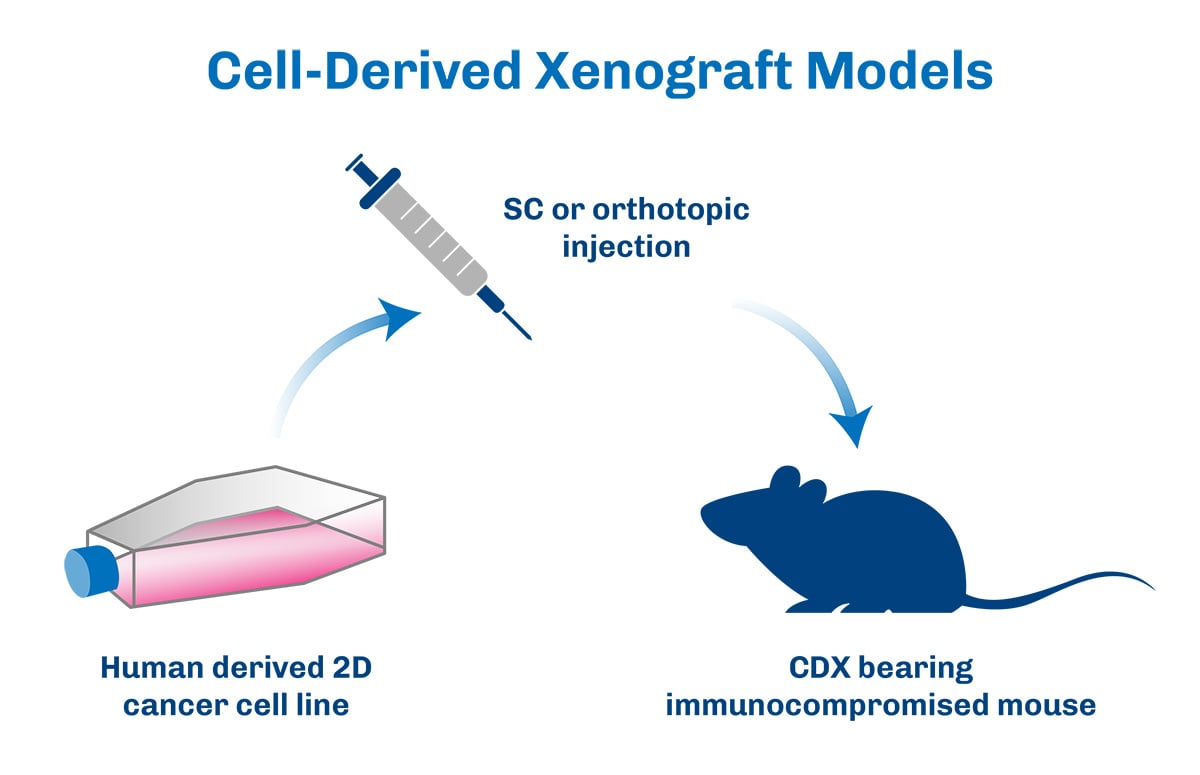
In oncology research, the ability to accurately predict clinical outcomes based on preclinical studies is critical for the successful development of new therapies. Cell Line Derived Xenografts (CDX) models have emerged as a powerful tool in this regard, providing robust platforms that bridge the gap between preclinical and clinical research.
Enhancing Clinical Predictive Value and Building Confidence
CDX models are designed to closely mimic the human tumor environment, making them highly relevant for studying cancer biology and therapeutic responses. These models are thoroughly validated for genetic fidelity, tumor growth characteristics, and responsiveness to standard treatments. This thorough validation ensures that the data generated from CDX models is both reliable and applicable to clinical settings. The predictive value of CDX models allows researchers to move into clinical trials with greater confidence in their therapeutic candidates. By providing robust preclinical data, these models help to de-risk the transition to clinical stages, thereby supporting the development of more effective cancer therapies.
Understanding Tumor Biology and Detailed Therapeutic Evaluation
CDX models offer a comprehensive understanding of tumor biology, treatment responses, and resistance mechanisms. By replicating human tumor characteristics in a controlled environment, these models allow for in-depth study of tumor growth kinetics, metastatic spread, and interaction with the host environment. This detailed therapeutic evaluation enables researchers to gain insights into tumor response by observing changes in tumor size, growth rate, and other parameters. This detailed visualization of tumor dynamics is necessary for assessing the efficacy of therapeutic candidates and making informed decisions about their potential clinical applications.
Leveraging Expertise and Technology
The integration of advanced technologies with CDX models enhances their predictive power. High-resolution imaging, sophisticated data analysis tools, and genetically validated models work together to provide a comprehensive understanding of tumor biology and treatment responses. Researchers can leverage these technologies to design more effective preclinical studies and generate high-quality data that supports clinical decision-making.
Conclusion
The ability to accurately predict clinical outcomes based on preclinical data is crucial for advancing oncology research. CDX models provide strong predictive value, helping researchers bridge the gap between preclinical studies and clinical trials. By offering detailed therapeutic evaluation and insights into tumor response, these models support the development of more effective cancer therapies.
To learn more about how advanced imaging capabilities and CDX models can enhance your preclinical oncology research, visit TD2’s webpage: Cell Line Derived Xenografts (CDX) for Preclinical Studies