The evolving complexity of oncology drug development demands an equally sophisticated approach to understanding the tumor microenvironment and its response to therapeutic interventions. Multi-omic screening, encompassing proteomics, metabolomics, transcriptomics, and genomics, has emerged as a powerful tool for gaining comprehensive insights into tumor biology. In this blog, we focus on the importance of incorporating metabolomics and proteomics into preclinical oncology studies and how this data can guide critical decision-making in drug development.
Why Multi-Omic Screening Matters in Oncology
Cancer is a multi-faceted disease influenced by genetic mutations, epigenetic changes, and dynamic biochemical interactions. Traditional approaches, such as genomics or transcriptomics alone, often fail to capture the functional and phenotypic complexity of tumor biology. Multi-omic screening bridges this gap by integrating data from various biological layers, enabling a holistic understanding of tumor systems.
Metabolomics and proteomics are essential for exploring the functional consequences of genetic and epigenetic alterations, providing actionable insights into tumor metabolism, signaling pathways, and drug responses.
The Role of Metabolomics in Preclinical Oncology
Metabolomics is the study of small-molecule metabolites within cells, tissues, or organisms. These metabolites serve as the end products of cellular processes and provide real-time snapshots of tumor metabolism and its interaction with therapeutic agents. Key applications of metabolomics in preclinical oncology include:
- Identifying Tumor-Specific Metabolic Pathways
Cancer cells often exhibit altered metabolic pathways, such as the Warburg effect or glutamine addiction, to support rapid growth. Metabolomic profiling can identify these unique metabolic signatures, which can then be targeted by therapeutic agents. - Evaluating Drug Efficacy and Mechanisms of Action
Metabolomics can reveal changes in metabolic profiles in response to treatment, helping researchers understand whether a drug effectively disrupts tumor metabolism. For example, inhibitors of glycolysis can be validated by monitoring lactate production in tumor models. - Uncovering Biomarkers for Early Drug Response
Changes in metabolite levels can act as early indicators of treatment efficacy or resistance, providing critical data to guide go/no-go decisions in drug development pipelines.
The Role of Proteomics in Preclinical Oncology
Proteomics focuses on the large-scale study of proteins, which are the functional drivers of cellular processes. Proteomic analyses provide a wealth of information about protein expression, post-translational modifications, and protein-protein interactions in cancer. Key applications include:
- Identifying Drug Targets and Resistance Mechanisms
Proteomic profiling can uncover differentially expressed or mutated proteins that drive tumor progression or confer resistance to therapy. For example, kinome profiling can identify upregulated kinases in resistant tumor cells, guiding the development of combination therapies. - Mapping Tumor Signaling Networks
Proteomics can delineate complex signaling networks that regulate tumor growth and immune evasion. Insights from these networks help predict off-target effects and refine therapeutic strategies. - Development of Predictive Biomarkers
Proteomic data can identify protein signatures associated with treatment response, enabling the stratification of patients most likely to benefit from a particular drug.
Integrating Multi-Omic Data in Drug Development
Incorporating metabolomics and proteomics into preclinical oncology studies creates a robust dataset that enhances decision-making throughout the drug development process:
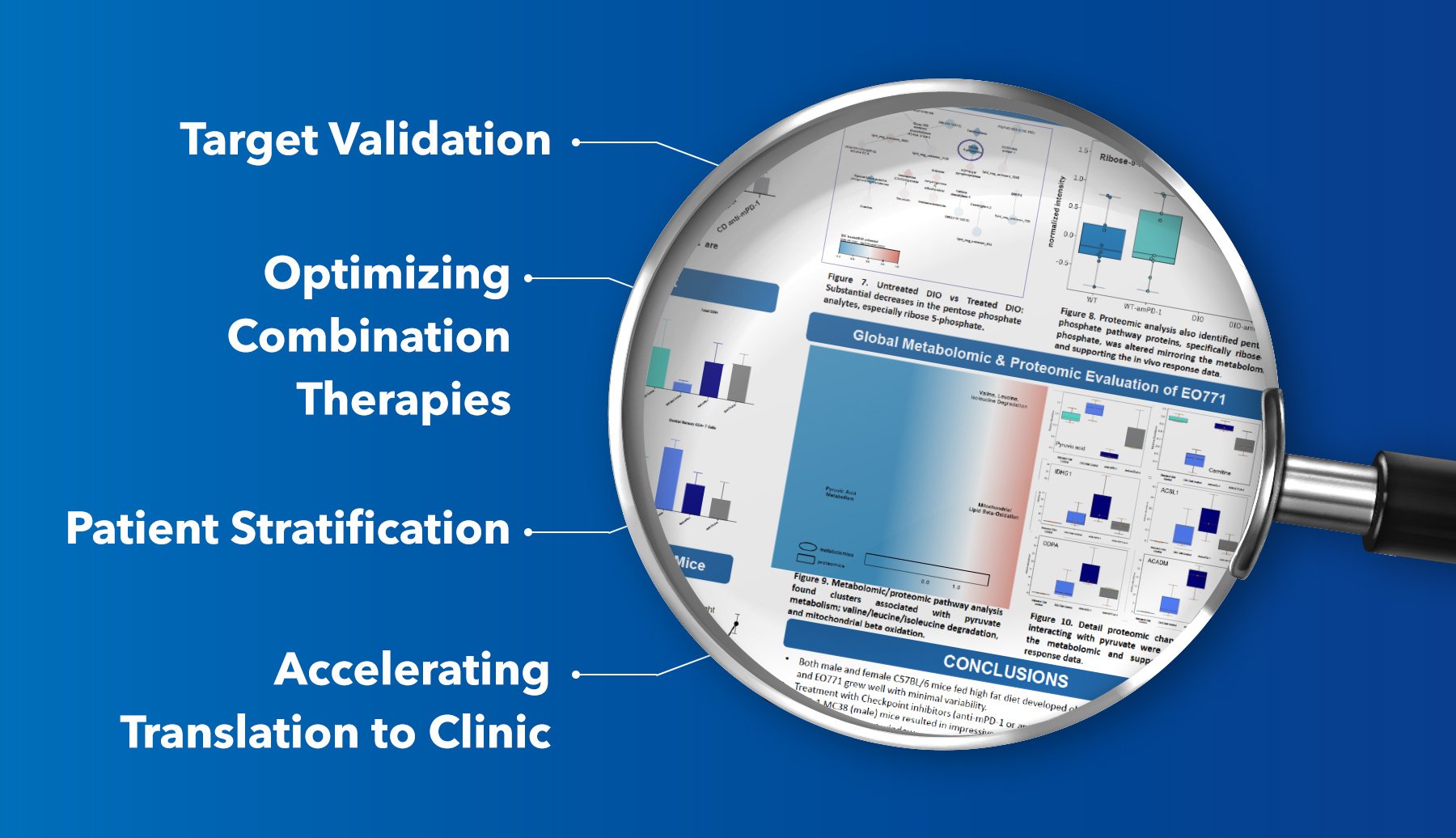
- Target Validation
By combining proteomic data with metabolomic signatures, researchers can validate the functional importance of a drug target, ensuring its relevance in disease progression. - Optimizing Combination Therapies
Multi-omic data can highlight complementary pathways or potential resistance mechanisms, guiding the selection of synergistic combination therapies. - Patient Stratification
Multi-omic profiles derived from in vivo and in vitro models can be translated to clinical settings, aiding in the identification of biomarkers for precision oncology. - Accelerating Translation to Clinic
Early identification of pharmacodynamic biomarkers through multi-omics reduces the uncertainty associated with preclinical-to-clinical translation, ultimately de-risking drug development.
Case Study: Multi-Omic Insights from Male and Female DIO Mouse Models in Checkpoint Inhibitor Studies
A recent study presented by Translational Drug Development at the 2024 SITC conference demonstrates the critical role of multi-omic screening in uncovering gender-specific and metabolic influences on checkpoint inhibitor efficacy (TD2-Poster-Abstract-1364).
Study Overview:
The study investigated the antitumor efficacy of checkpoint inhibitors (anti-PD-1 and anti-CTLA-4) in diet-induced obese (DIO) male and female mice using syngeneic tumor models: MC38 murine colon carcinoma in males and EO771 murine breast carcinoma in females. Researchers compared these results to control diet (CD) mice to evaluate the impact of obesity-induced metabolic changes on treatment outcomes.
Key Findings:
- Enhanced Antitumor Activity in DIO Mice:
- Male DIO mice (MC38 model) treated with anti-PD-1 exhibited robust tumor growth inhibition (TGI = 53%) compared to no measurable response in CD mice.
- Female DIO mice (EO771 model) showed impressive responses to both anti-PD-1 and anti-CTLA-4 treatments, achieving over 70% TGI and tumor stagnation during the treatment period. CD mice showed no response (TGI = 0%).
- Gender-Specific Tumor Microenvironment (TME) Differences:
Flow cytometry analysis revealed marked differences in immune cell populations between male and female models, including variations in CD4+ and CD8+ T-cell proportions. These differences highlight the need for gender-specific evaluation in preclinical oncology studies. - Metabolomic and Proteomic Insights:
- Metabolomic profiling uncovered shifts in glycolysis and pentose phosphate pathways, with notable changes in ribose 5-phosphate levels, indicating altered metabolic dependencies in DIO models.
- Proteomic analysis further identified pathway alterations in mitochondrial beta-oxidation and pyruvate metabolism, correlating with enhanced therapeutic responses in DIO mice.
Poster Conclusion:
This study demonstrates that DIO models provide a more clinically relevant platform for evaluating immunotherapies, particularly when considering gender-specific and metabolic influences. By integrating metabolomics and proteomics, researchers can gain nuanced insights into the interplay between obesity, immune responses, and therapeutic outcomes, enabling the development of targeted interventions for diverse patient populations.
Conclusion
The integration of metabolomics and proteomics into preclinical oncology studies is no longer a luxury—it is a necessity. By providing a detailed understanding of tumor biology at functional and biochemical levels, these tools enhance the predictive power of in vivo and in vitro models. Multi-omic screening not only accelerates the drug discovery process but also ensures that preclinical findings are translatable to clinical success. For oncology researchers, investing in multi-omic capabilities represents a crucial step toward developing more effective and targeted cancer therapies.