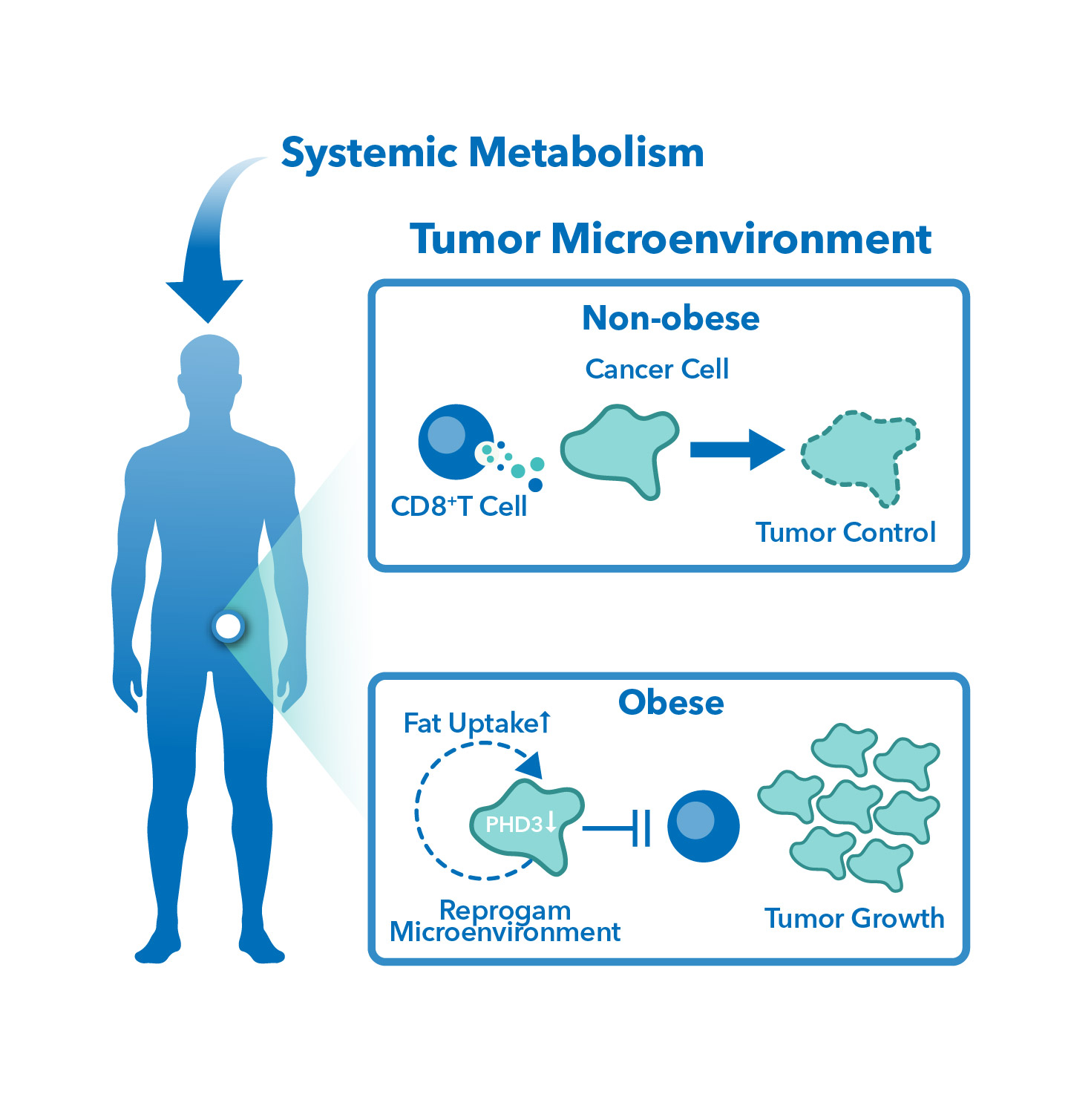
Obesity is a growing health concern worldwide. According to research, it is associated with an increased risk for cancer of at least 13 anatomic sites, including endometrial, esophageal, renal and pancreatic adenocarcinomas; hepatocellular carcinoma; gastric cardia cancer; meningioma; multiple myeloma; colorectal, postmenopausal breast, ovarian, gallbladder and thyroid cancers. Although the mechanism of this relationship is unclear, the existence of diet-based therapies (ketotherapy) as a form of cancer treatment suggests that diet somehow affects the activities of cancer cells or its environment. Preclinical studies are underway to gain a better understanding of how excess body weight affects the tumor microenvironment (TME) or how it impacts antitumor immunity.
Obesity and Cancer
One hypothesis is that obesity leads to a state of chronic inflammation in the body, thus increasing the risk of cancer development. Adipose tissue (fat cells) produces several pro-inflammatory cytokines, which can contribute to a chronic state of inflammation. Chronic inflammation can lead to the accumulation of DNA damage and mutations, which can promote the development of cancer.
It has also been reported that obesity alters hormone levels in the body, particularly sex hormones such as estrogen and testosterone, increasing the risk of hormone-related cancers such as breast and endometrial cancer. Adipose tissue also produces adipokines, which can affect insulin resistance and glucose metabolism, which are also factors that can contribute to cancer development.
Lastly, obesity can alter the tumor microenvironment (TME) by affecting the immune system. Adipose tissue produces several factors that can suppress the immune system, leading to decreased anti-tumor immune responses. Obesity can also lead to changes in metabolism, which can alter the way cells produce energy and affect tumor growth.
Combatting Obesity-triggered Cancer: Are we using the right models?
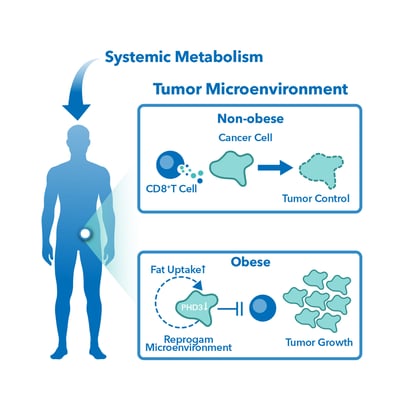 To gain a better understanding of how excess body weight affects the tumor microenvironment (TME) or how it impacts antitumor immunity, preclinical studies are underway, and they play a crucial role in drug development. The outcome of preclinical studies determines whether a drug will proceed to clinical trials. Hence, several factors need to be considered to add rigor and improve their quality. Unfortunately, most preclinical studies that indicated therapeutic effect on animals do not translate to studies in humans. One main reason is attributed to poor clinical relevance of in vitro and/or in vivo models.
To gain a better understanding of how excess body weight affects the tumor microenvironment (TME) or how it impacts antitumor immunity, preclinical studies are underway, and they play a crucial role in drug development. The outcome of preclinical studies determines whether a drug will proceed to clinical trials. Hence, several factors need to be considered to add rigor and improve their quality. Unfortunately, most preclinical studies that indicated therapeutic effect on animals do not translate to studies in humans. One main reason is attributed to poor clinical relevance of in vitro and/or in vivo models.
Traditionally, in vivo mouse models fed with controlled diets (CD) have been used in obesity-associated cancer studies. However, recent research has shown that diet-induced obesity (DIO) mouse models provide a more clinically relevant system to study the effect of obesity on cancer and evaluate the efficacy of cancer therapies. These models allow a better understanding of how obesity-induced systemic changes in metabolism affect the TME after treatment.
Several diet-based therapies, such as ketogenic diets, have also been investigated as a form of cancer treatment. The manipulation of glucose levels in cells through dietary manipulation alters the production of downstream metabolites related to tumor growth. Ketogenic diets have been shown to synergistically improve cytotoxic chemotherapy, implying the effect of diet on tumor growth.
TD2 specializes in improving the overall quality of preclinical studies. This includes establishing a diet-induced obesity (DIO) tumor mouse models from syngeneic cell lines to screen cancer therapies in a more clinically relevant system. Comparison between DIO and CD models showed significantly different body weight which could potentially affect the metabolism in the TME and other downstream effects.
Benefits of DIO mouse models
Using DIO mouse models in preclinical studies pose several advantages:
- They allow studies to be executed in a more clinically relevant model that is reflective of western patient populations.
- They allow a better understanding as to how obesity-induced systemic changes in metabolism affect the tumor microenvironment after treatment.
- They allow the evaluation of metabolite differences after treatment with immune checkpoint inhibitors and targeted agents.
Scientific Perspective of DIO Mouse Models
Cell survival requires ATP, a chemical form of energy. There are two ways to produce ATP: (1) cells utilize glucose in a process called glycolysis; and (2) cells use up NADH and oxygen in a process called oxidative phosphorylation. However, there is a limit to how much NADH a cell can handle. When this limit is reached, NADH accumulation occurs which ultimately leads to buildup of Reactive Oxygen Species (ROS).
High ROS is typically associated in tumors which is why cells avoid NADH accumulation. One way is the manipulation of glucose levels in cells which alters the production of downstream metabolites related to tumor growth. Hence, dietary manipulation is being investigated for its effect on tumor growth. Ketogenic diets, for instance, have been shown to synergistically improve cytotoxic chemotherapy implying the effect of diet on tumor growth.
Conclusions
Research suggests that obesity is associated with an increased risk of several types of cancer. While the mechanism behind this relationship is unclear, preclinical studies are underway to gain a better understanding of how excess body weight affects the tumor microenvironment or antitumor immunity. However, traditional in vivo mouse models fed with controlled diets may not accurately reflect the metabolic changes induced by obesity. The establishment of diet-induced obesity (DIO) mouse models provides a more clinically relevant system to study the effect of obesity on cancer and evaluate the efficacy of cancer therapies. Additionally, manipulating glucose levels in cells through dietary manipulation, such as ketogenic diets, has been shown to impact tumor growth and may have synergistic effects with cytotoxic chemotherapy.
Reference:
- https://www.cdc.gov/cancer/obesity/index.htm
- Avgerinos KI, Spyrou N, Mantzoros CS, Dalamaga M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metabolism. 2019 Mar;92:121-135. doi: 10.1016/j.metabol.2018.11.001. Epub 2018 Nov 13. PMID: 30445141.
- Strulov Shachar, S., & Williams, G. R. (2017). The Obesity Paradox in Cancer-Moving Beyond BMI. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 26(1), 13–16. https://doi.org/10.1158/1055-9965.EPI-16-0439
- Aban, I. B., & George, B. (2015). Statistical considerations for preclinical studies. Experimental neurology, 270, 82–87. https://doi.org/10.1016/j.expneurol.2015.02.024
- Yang L, TeSlaa T, Ng S, Nofal M, Wang L, Lan T, Zeng X, Cowan A, McBride M, Lu W, Davidson S, Liang G, Oh TG, Downes M, Evans R, Von Hoff D, Guo JY, Han H, Rabinowitz JD. Ketogenic diet and chemotherapy combine to disrupt pancreatic cancer metabolism and growth. Med (N Y). 2022 Feb 11;3(2):119-136. doi: 10.1016/j.medj.2021.12.008. PMID: 35425930; PMCID: PMC9004683.