Regulatory
Regulatory, Preclinical
Navigating the Preclinical-to-Clinical Transition for ADCs: Regulatory & Study Design Strategies
Regulatory, Clinical
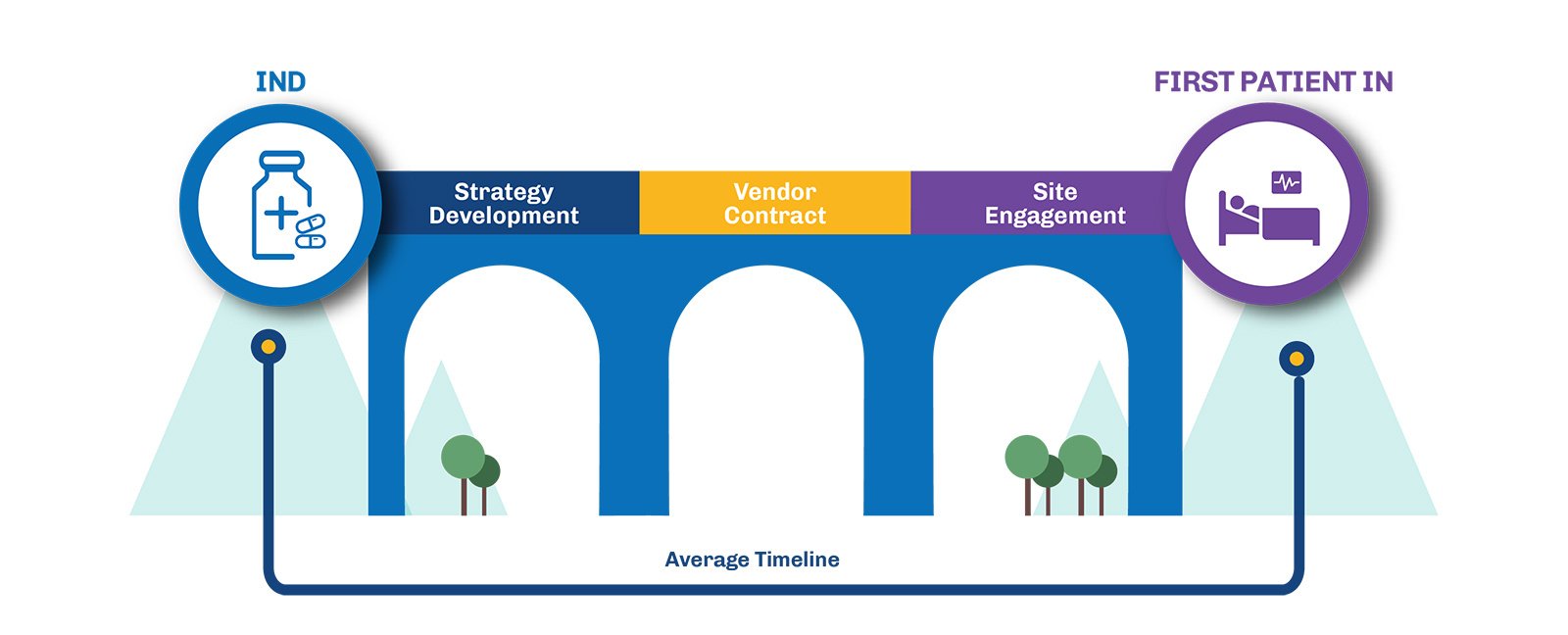
Navigating the Complexities of Clinical Trial Start-Up
Regulatory
Oncology Drug Approval Process
Regulatory