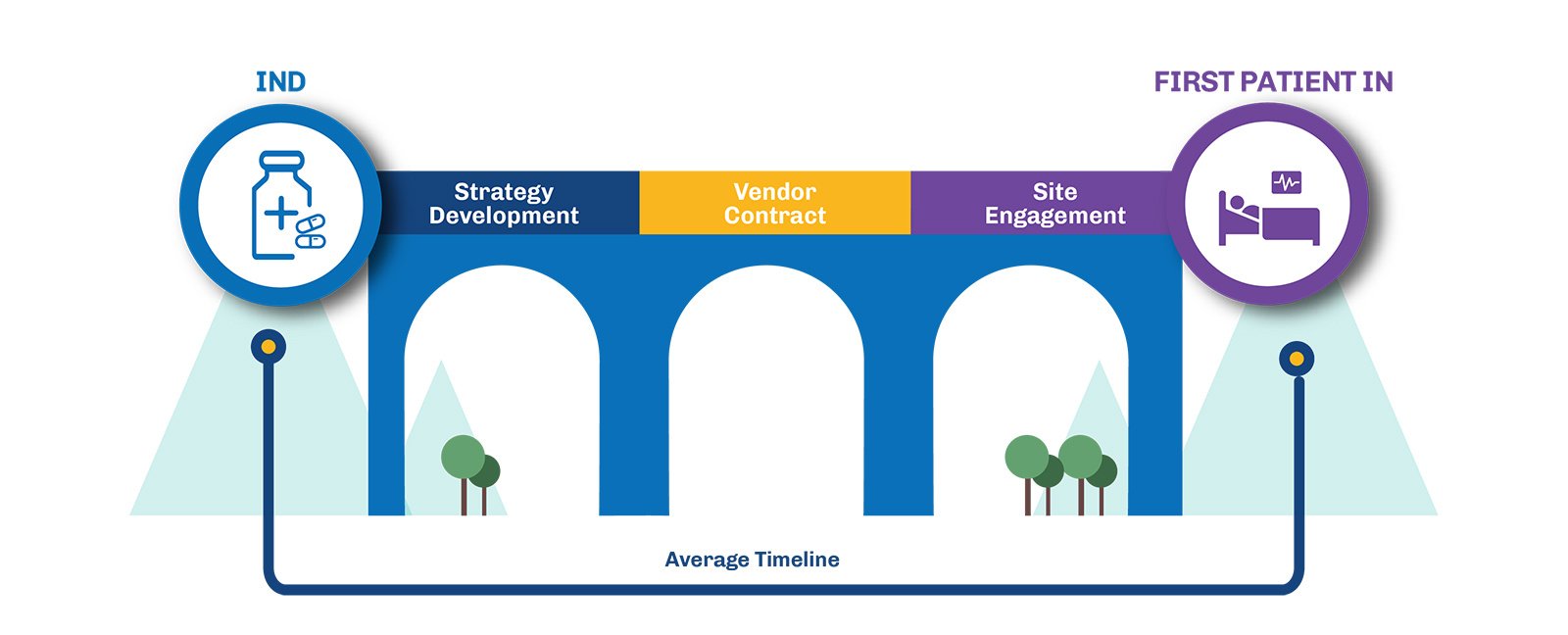
The Challenge: Overcoming Inefficiencies in Trial Start-Up
The period between receiving the FDA’s “May Proceed” letter and activating trial sites for patient enrollment often encounters delays that extend timelines and inflate costs. This phase is crucial for sponsors, who must navigate several hurdles to optimize the start-up process and bring treatments to patients faster.
Optimizing the Start-Up Process
To tackle these challenges, sponsors can adopt a more proactive approach to key start-up activities. Let’s explore how each step can be optimized to benefit researchers:
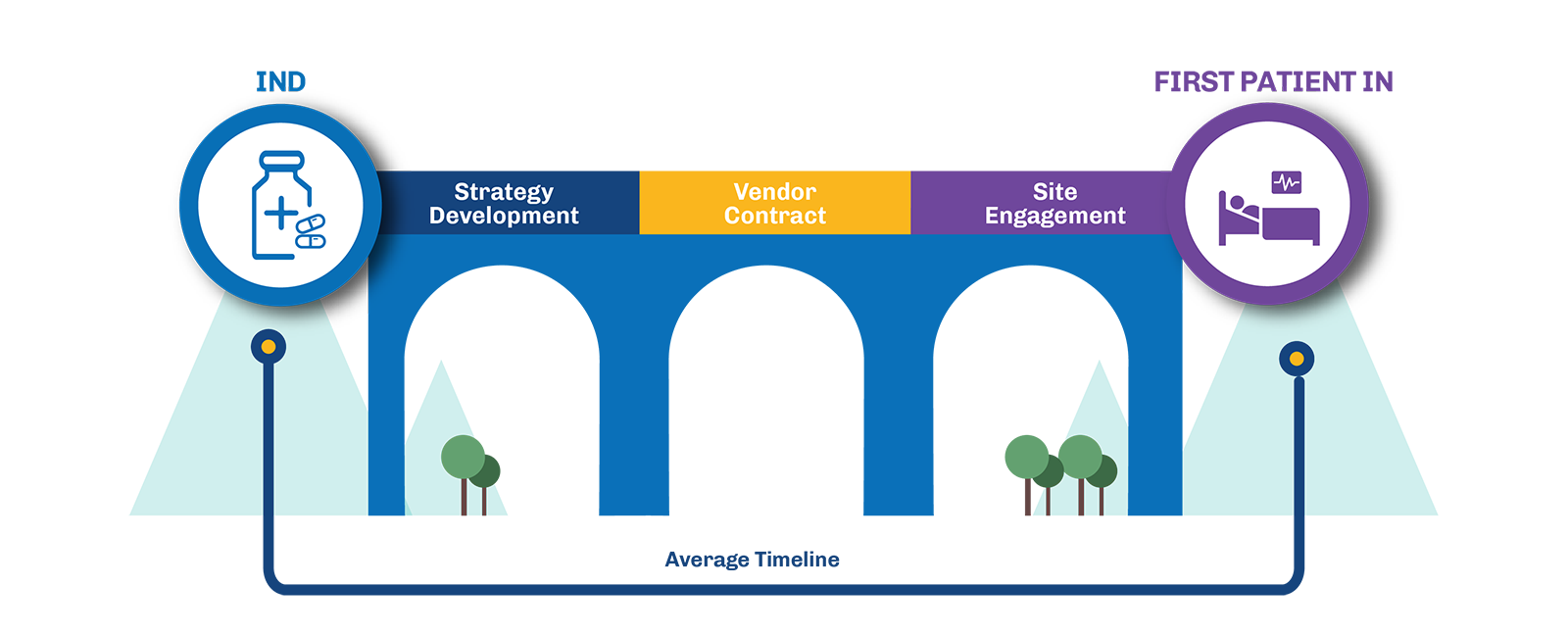
- Early Vendor Engagement: Securing vendor contracts before regulatory approval is essential to preventing delays later in the process. By locking in key services early, such as laboratory services, flow cytometry, imaging, drug depots, sponsors can ensure that everything is in place to move forward the moment the FDA gives the green light. This approach not only reduces the risk of bottlenecks but also allows researchers to focus on trial execution rather than scrambling for resources.
- Proactive Site Selection: Engaging with investigators and pre-selecting trial sites early in the regulatory process ensures that sites are ready to activate as soon as approval is granted. By identifying high-performing sites and establishing relationships with investigators ahead of time, sponsors can minimize the time spent on site initiation and maximize enrollment efficiency. This proactive strategy helps avoid the common pitfall of delayed site activation, which can add months to the trial timeline.
- Advanced Protocol Preparation: Finalizing protocols and essential documents before receiving the “May Proceed” letter enables sponsors to move forward quickly once regulatory clearance is obtained. Early protocol development includes aligning the study design with regulatory expectations, anticipating potential challenges, and preparing informed consent forms and Investigator Brochures. This thorough preparation not only speeds up the trial start-up but also ensures that the study is scientifically robust and compliant with regulatory requirements.
The Impact: Accelerating Timelines and Reducing Costs
By implementing these proactive strategies, sponsors can accelerate site activation to just 6-8 weeks and enroll the first patient up to 4+ months sooner. This approach not only expedites the clinical trial process but also reduces oversight costs, making the trial more efficient and cost-effective.
Conclusion: A Strategic Approach to Clinical Trial Start-Up
Streamlining the clinical trial start-up process requires strategic planning and early engagement. By addressing the common inefficiencies in this phase, sponsors can reduce timelines, minimize costs, and ultimately deliver new treatments to patients faster.
Learn more about optimizing your clinical trial start-up with TD2’s Clinical Trial Enablement Program.