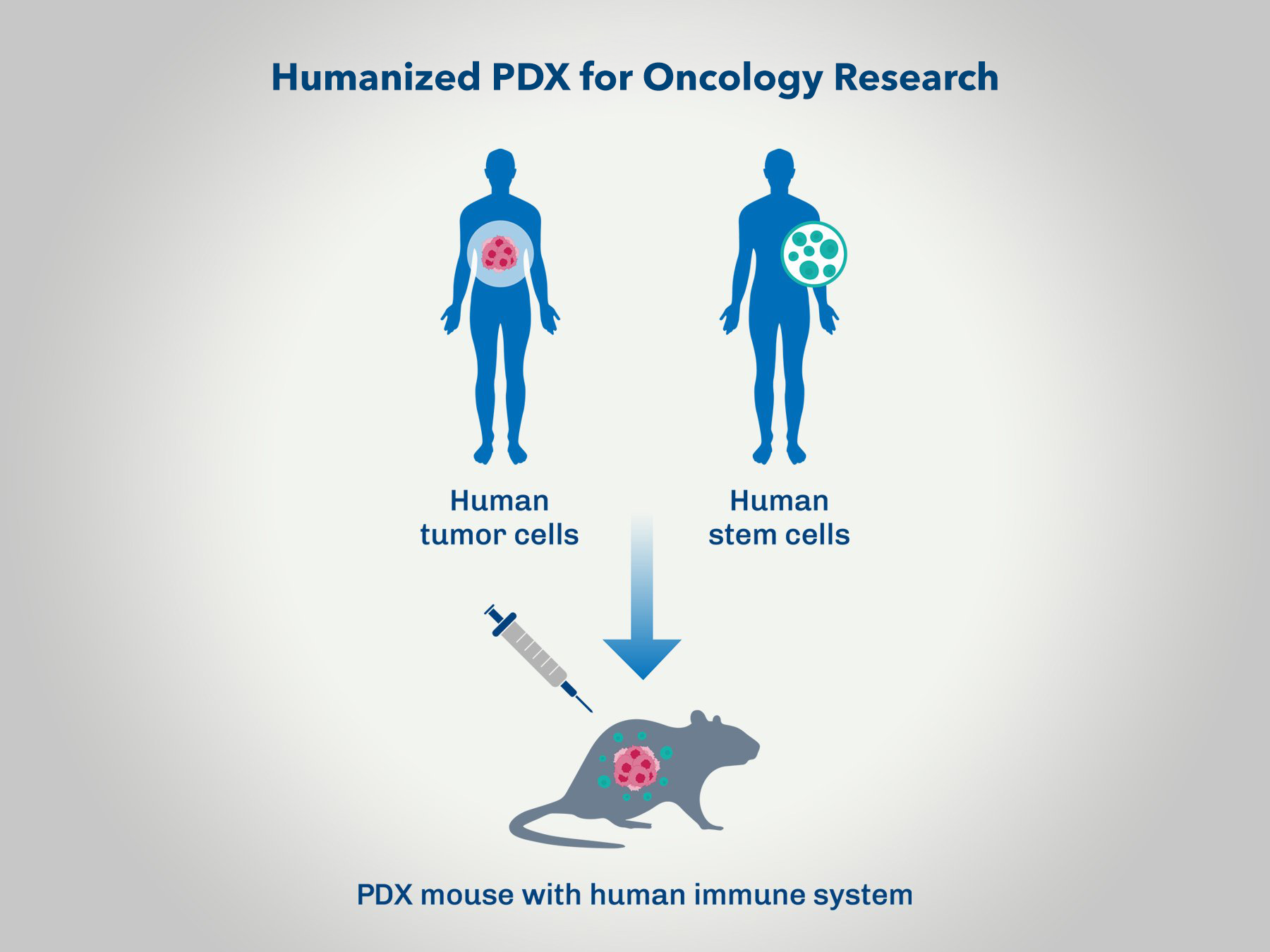
Preclinical oncology studies are a critical step in developing effective cancer therapies. Patient-derived xenograft (PDX) models have become a cornerstone of these efforts, offering high fidelity in replicating human tumor biology in vivo. However, one of the persistent limitations of traditional PDX models is their lack of a functional human immune system, which restricts their utility in evaluating immuno-oncology therapies. Recent advancements in humanized immune system (HIS) models have transformed this landscape, enabling researchers to gain deeper insights into the complex interactions between tumors and the immune system.
Here, we explore why conducting your next PDX oncology study in a humanized immune system is essential for advancing cancer research and therapeutic development.
The Gap in Traditional PDX Models: A Lack of Immune Context
Traditional PDX models rely on immunodeficient mice, which allow for the engraftment and growth of human tumors without rejection. While this enables the study of tumor biology and response to standard therapies, it inherently excludes the immune system's role in tumor progression and therapy resistance. This limitation is particularly critical for immuno-oncology research, where therapies such as immune checkpoint inhibitors, CAR-T cells, and bispecific antibodies depend on functional interactions with the immune system.
Without the immune context, traditional PDX models fail to:
- Predict immune-mediated therapeutic efficacy.
- Assess the impact of therapies on immune cell populations.
- Model immune escape mechanisms and resistance.
Humanized Immune System Models: Bridging the Gap
Humanized mouse models are engineered to recapitulate components of the human immune system within a murine host. This is typically achieved by engrafting immunodeficient mice with human hematopoietic stem cells (CD34+ HSCs) or peripheral blood mononuclear cells (PBMCs). These models develop functional human immune cell populations, including T cells, B cells, natural killer cells, and macrophages, providing a robust platform for studying immune-tumor dynamics.
Key advantages of HIS models in PDX studies include:
- Immuno-Oncology Therapy Evaluation: HIS models allow researchers to test therapies that rely on immune activation, such as immune checkpoint inhibitors (e.g., anti-PD-1, anti-CTLA-4) or bispecific T-cell engagers.
- Immune-Tumor Crosstalk: HIS models enable the study of mechanisms like immune suppression, tumor immune evasion, and cytokine signaling, which are critical for understanding therapeutic resistance.
- Biomarker Discovery: HIS models facilitate the identification of immune-related biomarkers that predict response or resistance to therapies, aiding in patient stratification.
- Preclinical-Clinical Translation: By better replicating human immune responses, HIS models enhance the translational relevance of preclinical findings.
Applications in Oncology Research
Checkpoint Inhibitors
Checkpoint inhibitors have revolutionized cancer therapy, but their efficacy is highly patient-specific, with some tumors exhibiting primary or acquired resistance. HIS-PDX models allow researchers to:
- Study the mechanisms of resistance.
- Test combinations of checkpoint inhibitors with other agents.
- Evaluate immune-related adverse events.
Cell-Based Therapies
CAR-T cells and TCR-engineered T cells require a functional immune system to evaluate their activity. HIS-PDX models provide a platform for assessing:
- Tumor-specific T-cell trafficking and infiltration.
- Cytokine release and associated toxicities.
- Efficacy against solid tumors, which are notoriously challenging for CAR-T therapies.
Bispecific Antibodies
Bispecific antibodies that engage T cells and tumor antigens can be evaluated for their ability to induce cytotoxicity in HIS models, providing a more accurate prediction of clinical outcomes.
Challenges and Considerations
While HIS models offer significant advantages, they are not without challenges. Researchers should be aware of:
- Graft-Versus-Host Disease (GVHD): PBMC-engrafted models are prone to GVHD, which can limit study duration.
- Incomplete Immune Reconstitution: CD34+ HSC models require time for immune system development, and some cell types (e.g., regulatory T cells) may be underrepresented.
- Human-Murine Interactions: Cross-species differences in cytokines and other immune mediators can complicate data interpretation.
Overcoming these challenges requires careful model selection, experimental design, and validation to ensure reproducibility and translatability.
Conclusion: Enhancing the Predictive Power of Preclinical Studies
Incorporating a humanized immune system into your next PDX oncology study offers unparalleled opportunities to evaluate the intricate interplay between tumors and the immune system. As the field of immuno-oncology continues to evolve, HIS-PDX models will be instrumental in bridging the gap between preclinical research and clinical application. By leveraging these models, researchers can more accurately predict therapeutic efficacy, uncover mechanisms of resistance, and identify biomarkers for patient stratification, ultimately accelerating the development of life-saving cancer therapies.