By Jennifer J. Stewart PhD
The Clinical and Laboratory Standards Institute (CLSI) is a nonprofit organization that develops and publishes standards that are accredited by the American National Standards Institute (ANSI) and are referenced in government regulations and international standards. As a volunteer-driven, membership-supported organization, CLSI promotes documents on a variety of specialty areas, from laboratory basics to quality management systems, verification and validation, and information management. CLSI uses a consensus process (described in the figure below) that brings together experts from industry, government, and health care professions to ensure balanced representation to develop timely standards that can be confidently adopted by laboratories, industry, accreditors, and regulators as the way to improve laboratory testing.
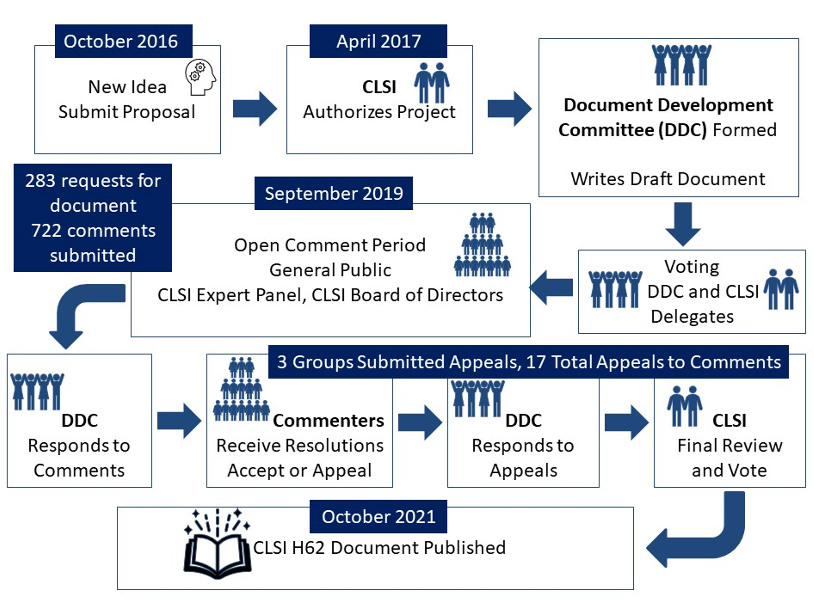
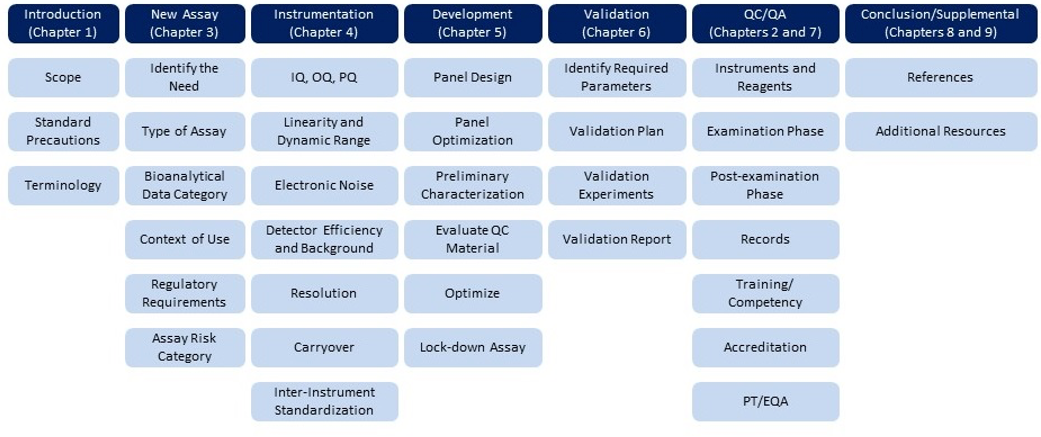
On October 27, 2021, the first edition of CLSI H62–Validation of Assays Performed by Flow Cytometry was published. This document focuses on the unique requirements for the analytical validation of flow cytometry assays on cell-based systems. Recommendations are provided for pre-examination phase activities such as sample requirements, reagent optimization evaluation, instrument qualification and standardization, and assay optimization and validation. Guidance for examination phase activities such as instrument monitoring and QC are described, as are recommended practices for post-examination activities, including data review, reporting, storage, and retention. This guideline is intended for use in a flow cytometry environment in which preclinical (or nonclinical) and clinical assessments are conducted. The document covered in detail the topics listed below.
Representatives from TD2 were proud members of the committee that brought forth and critically reviewed the CLSI H62 document. Jennifer J Stewart was an author on Chapter 5 Assay Development and Optimization and a main reviewer for Chapter 6 Analytical Method Validation. Additionally, TD2 staff contributed to the open comment period.
TD2 is a proud contributor to many white papers and guidance documents defining the direction of the field. As a contract flow lab, TD2 provides high throughput and high-capacity flow cytometry services, running multiple flow cytometers with multiparameter antibody panels daily. We are proficient in processing a multitude of specimen types including whole blood, frozen PBMCs along with cell culture and tissue processing capabilities. Our flexibility in handling so many specimen types allow for the support of a wide range of flow cytometry assays including: immunophenotyping/lymphocyte subset analysis, receptor occupancy, functional assays, and cell viability/apoptosis measurements. Our expert staff is always available to help guide you through these tests, and we welcome clients to visit our facility. We encourage sponsor engagement throughout the process. Contact us for more information!



